For PFAS, background matters!
This is the first installment in a series of postings on per- and polyfluoroalkyl substances – PFAS. PFAS are a class of widely-used human-made chemicals now viewed by many as a threat to human health, and a recent focus of the environmental industry. This initial discussion considers the presence and transport of PFAS in the environment with a focus on anthropogenic background of PFAS in soil.
Trite but seemingly true, PFAS are ubiquitous – not only in myriad products and applications – but in the environment as well, albeit typically in very low concentrations. Use of PFAS for decades, allowed both direct and indirect releases of PFAS to soil, water, and air. Consequently, PFAS – especially the environmentally stable perfluorinated compounds (PFCs) – have distributed throughout the environment. PFAS concentrations in excess of drinking water standards are typically the result of specific sources, but the general background presence of PFAS is sometimes important to consider.
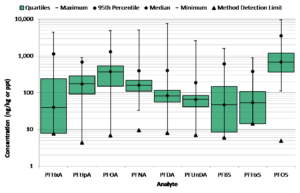
Sanborn Head collaborated on a University of Vermont study that investigated the background presence of PFCs in shallow soils. Samples were collected from 66 Vermont locations near no known sources of PFAS – e.g., town greens and state parks. PFCs were detected in each sample at low concentrations, generally on the order of one to several parts per billion. Concentrations of the most frequently detected PFCs in the study are illustrated in the following “box and whiskers” diagram.
Implications of Widespread PFAS Detection in Soils
The widespread detection of PFAS in VT soils has important implications. Assuming these results reflect regional background conditions, PFAS should be detectable in most soil samples given the low reporting limits now achievable by commercial laboratories. Hence, PFAS detected in soil might not be due to a local release. Additionally, regulatory standards in soil designed to protect groundwater must incorporate PFAS background. As a recent example, the Massachusetts Department of Environmental Protection (MassDEP) initially proposed a soil standard lower than the background levels measured in VT soils based on traditional partitioning models that likely overpredict PFAS leaching from soil to groundwater. In response to comments, MassDEP raised and issued its standard above typical background levels. This example illustrates the potentially significant implications of failing to incorporate background concentrations into soil regulatory standards, which could conceivably require response actions at PFAS sites at levels lower than anthropogenic background, and also shift limited resources from other environmental concerns.
Reliable models for predicting PFAS leaching from soil are lacking at present, but there appears to be a tendency for longer-chain PFAS to stick to soil, making it a long-term reservoir for potential groundwater contamination. Based on a simple mass balance, a concentration of 1 part per billion (ppb) in soil (equal to sum of the median levels of PFOA and PFOS found in VT soils) is sufficient to supply a concentration of 20 parts per trillion (ppt) to an equivalent depth of groundwater 75 times1. Fortunately, rates of PFAS leaching from background soils appear to be low, based on a lack of evidence of widespread detection of PFAS in groundwater at ppt levels. The majority of groundwater samples collected outside of sites are either non-detect or below stringent State regulatory standards in the 10-20 ppt range.
PFAS in the Environment: Atmospheric Transport
As a final thought, the detection of PFAS at low levels in VT soils implies that atmospheric transport and deposition have an important role in distributing PFAS throughout the environment. The saying “what goes up, must come down” is especially true for stable PFAS since current data indicate that these compounds do not degrade. Presumably the presence of compounds such as PFOA and PFOS in soil reflects mostly legacy releases prior to the voluntary ban on their production. Atmospheric deposition may still play a role in the fate of PFAS in the global environment, perhaps in more complex ways. Conjecturally, the fluorotelomer alcohols (FtOHs) detected in ambient air sampling studies likely reflect emissions of these compounds, which have replaced PFOA and PFOS in many applications. FtOHs have been shown to degrade to stable PFAS in the environment. Hence, the atmospheric transport and deposition of FtOHs and/or their stable PFAS degradants may be continuing to distribute PFAS throughout the environment.
I and my colleagues at Sanborn Head have been considering PFAS background and atmospheric deposition in our project work in efforts to distinguish localized PFAS releases from regional background. For further information on these topics, please contact us, and/or click on the following link to provide access to a copy of the Vermont soil PFAS background study, and recent presentations on atmospheric deposition of PFAS and PFAS behavior in soils (partitioning and leaching). Also stay tuned for the next segment, which will focus on PFAS in groundwater and surface water.
For Steve’s full presentation on PFAS Fate & Transport, click the video below!
1Assuming a bulk soil density of 1.5 g/cm3 for soil and water density of 1 g/cm3, 1 ppb = 1,000 ng/kg in soil can supply 20 ppt = 20 ng/kg = 20 ng/L in an equivalent depth of groundwater 1,000×1.5÷20 = 75 times.
Posted In: Articles
Tagged In: Industrial, PFAS, Research





