Per- and polyfluoroalkyl substances, known as PFAS, are the most well-known emerging contaminants and have become an urgent environmental issue in recent years. PFAS have been detected in landfill leachate at levels above drinking water standards. Although PFAS treatment is not currently required for landfill leachate, the progress in PFAS regulation at the national level suggests that PFAS standards in surface water will be established and landfill leachate treatment will be required.
As landfill operators contemplate changes in environmental regulations, it should be noted that not all PFAS contaminants behave in the same way. In fact, examining the compound chain length of PFAS will help us to identify which compounds require additional treatment to eliminate their impacts on the environment.
PFAS Chain Length Impact on Contamination
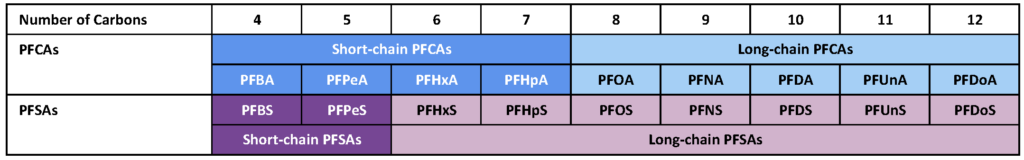
Based on the carbon chain number, perfluoroalkyl acids (PFAAs) can be divided into short-chain and long-chain compounds, as shown in Table 1. The classification of short- and long-chain PFAS is based on the bioaccumulation potential of the individual compound. The long-chain PFAS typically show high bioaccumulation potential. For perfluoroalkyl carboxylic acids (PFCAs), with a carboxylic function head, the long-chain PFAS include compounds with a carbon number of more than seven. For perfluoroalkyl sulfonic acids (PFSAs), with a sulfonic function head, the long-chain PFAS include compounds with a carbon number of more than five. The chain definition of polyfluoroalkyl substances is not well defined but generally follows a similar trend as PFAAs.
Landfill Leachate Composition
Half of the total PFAS in landfill leachate can be contributed by polyfluoroalkyl substances, especially 5:3 Fluorotelomer carboxylic acid (5:3 FTCA) – a compound not on most analytical lists. However, typically only PFAAs are regulated and most landfills have been collecting PFAAs and a limited number of polyfluoroalkyl substances. Figure 1 demonstrates the PFAAs composition in an example landfill. At either the upper or lower limits of the total concentration range, the short-chain PFAS are the dominant group. Although perfluoroheptanoic acid (PFHpA) is listed as short-chain in Table 1, its characteristic is in-line with long-chain and grouped with long-chain PFAS.
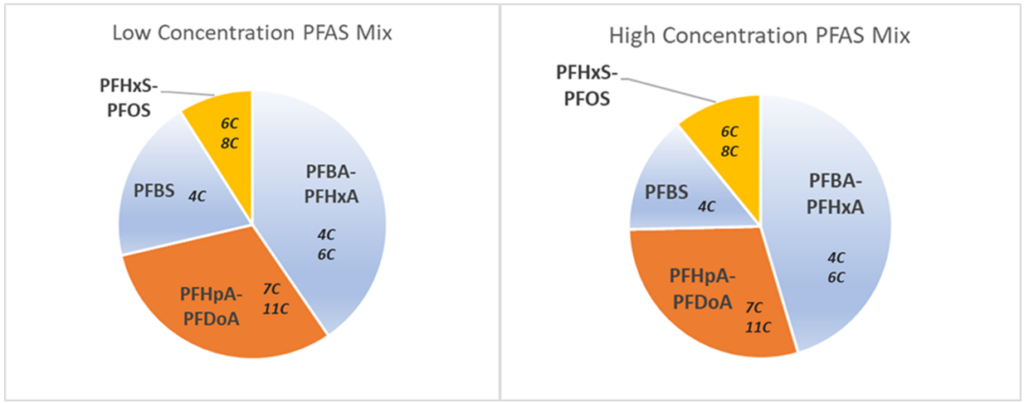
Left – Composition at lowest combined concentration; Right – Composition at highest combined concentration
PFAS Chain Length on Chemical and Physical Characteristics
All PFAS, being surfactants, contain a hydrophobic tail and a hydrophilic function head. Long-chain PFAS show more hydrophobicity (dislike water) and short-chain PFAS show more hydrophilicity (like water). Therefore,
- Higher solubility is often observed for a compound with high hydrophilicity. Solubility typically increases when the carbon chain number decreases.
- Sorption efficiency is high for a compound with high hydrophobicity. Sorption typically increases when the carbon chain number increases.
- Surfactants can form foam when gas is applied to the water. The ability to form foam increases when the carbon chain number increases. Short-chain PFAS are less effective at forming foams.
- PFAS also demonstrate volatility and short-chain PFAS can have higher volatility.
Impact on Regulation
A number of states have established groundwater and drinking water standards for PFAS in the low parts-per-trillion range, at levels 2-3 orders of magnitude lower than concentrations found in landfill leachate. Most of the state standards typically have targeted long-chain PFAS, which are known to bioaccumulate in humans in blood. However, the focus is shifting to include short-chain compounds as well, as evidenced by the recent Health Advisory Levels set by US EPA for Perfluorobutane sulfonic acid (PFBS) and GenX. Surface water standards will follow and will likely be set at levels equal to or lower than drinking water standards based on criteria to protect human health (and drinking water supplies). In anticipation, regulators are looking to establish effluent limitations under the NPDES program, and landfills that send leachate to wastewater treatment plants should anticipate limits on PFAS and/or pretreatment requirements.
Impact on Treatment
PFAS treatment is challenging due to the physical and chemical character of PFAS and the landfill leachates. Further, all available treatment technologies are generally less effective for short-chain PFAS. Note that none of the technologies can readily achieve the recently recommended levels from the national regulatory progress.
- Sorption: Three common sorption media includes activated carbon, ion-exchange (IX) resin, and organoclay. All sorption media can sequester PFAS via hydrophobic sorption. The sorption of short-chain PFAS is less effective because they generally show less hydrophobicity. IX and organoclay can also sorb PAFS via electrostatic charges. Therefore, IX and organoclay show higher sorption efficiency for short-chain PFAS, when compared with activated carbon. However, the high total organic carbon (TOC) and high total dissolved solids (TDS) in landfill leachate can reduce the sorption capacity of PFAS, especially for short-chain PFAS. High TOC competes for hydrophobic sorption and high salinity competes for electrostatic charge sorption.
- Reverse Osmosis (RO): RO filters water through semi-impermeable membrane under high pressure. Larger molecules typically can be more readily filtered. Long-chain PFAS are larger molecules than short-chain PFAS. Although RO can effectively filter all PFAS, the filtration efficiency of the short-chain is generally less than the long-chain. Because RO is prone to fouling and clogging, extensive pre-treatment processes are required to remove all suspended particulates (TSS), TOC, metals, and TDS.
- Precipitation: PFAS can be precipitated via coagulation & flocculation, the conventional wastewater treatment technology. PFAS generally show negative electrostatic charges at circumneutral pH conditions; therefore, cationic flocculant is more suitable for PFAS precipitation for landfill leachate with a circumneutral pH range. PFAS precipitate through electrostatic charge sorption with cationic flocculant and also co-precipitate with flocs. Higher hydrophobicity of PFAS results in more removal efficiency via co-precipitation. Therefore, short-chain PFAS are typically less effective for precipitation as well. In addition, high TOC and high salinity in leachate can also reduce the precipitation efficiency when compared with the typical groundwater treatment efficiency.
- Foam Fractionation: This technology removes PFAS using the ability of PFAS to form foams. This technology requires minimal pre-treatment and is not interfered by high TOC. Although high TDS can reduce the foamability of PFAS, foam can be formed and recovered as a waste concentrate, as shown during the site demonstrations at several landfill sites. However, the foamability of short-chain PFAS is significantly less than long-chain PFAS; therefore, this technology removal efficiency decreases with carbon chain number.
- Oxidation: All oxidation methods initially break down long-chain PFAS to short-chain PFAS, which can be considered as transient oxidation daughter products. Therefore, short-chain PFAS can be the most persistent PFAS in the oxidation treatment process. Many innovative methods using oxidation can be highly interfered by the high organic contents in the landfill leachate. The high persistence of the short-chain PFAS would likely occur if the TOC level is high in the landfill leachate.
Summary
The question is not whether landfills will need to treat landfill leachate for PFAS, but rather when treatment will be required, and what the design goals will be in terms of the specific PFAS and concentration limits. A variety of techniques are being explored for treating leachate, ranging from established absorption media (activated carbon and resins) to promising methods such as foam fractionation. Treatment systems will be landfill/leachate-specific and may involve bootstrapping of methods to optimize high removal efficiencies and cost effectiveness. An important unknown is whether regulations will stay focused on the long-chain, bioaccumulative PFAS, or whether short-chain compounds will need to be considered as well. If the latter case, costs could rise dramatically if treatment for short-chain compounds is needed, and resilient designs may be prudent in anticipation of future changes to the PFAS regulatory landscape.
Posted In: Articles
Tagged In: Leachate, PFAS, Research, Solid Waste